You’ve likely heard of SB 212 if you deal with pharmaceuticals or sharps waste. It stands for California’s Pharmaceutical and Sharps Waste Stewardship law (SB 212, Jackson, Chapter 1004, Statutes of 2018)
This law impacts many manufacturers or other members of the supply chain (also known as “covered entities”) who sell sharps as well as prescription and non-prescription drugs (also known as “covered drugs”) in California.
SB 212 requires covered entities to implement stewardship programs to properly collect and dispose of unused covered drugs and home-generated sharps waste. This can be done either individually, or through a collective “stewardship organization”.
The law focuses on providing convenient disposal options for “ultimate users.” These include:
- diabetes patients,
- those who inject a medicine with a needle,
- or anyone with unused medication.
SB 212 does not construct a take-back system for small businesses that generate sharps or pharmaceutical waste. These are governed by separate laws. (Don’t worry, we’ve got a solution to help businesses properly dispose of their sharps or needle waste at the end of this article.)
Many Are Already on Board
This Safe Needle Disposal.org list shows entities who are already on board with offering a take-back solution for their product users, such as offering a mail-back solution for medical waste.
Ultimately, SB 212 provides an easy, free way for ultimate users to properly dispose of their covered drugs and home-generated sharps waste. The covered entities need to outline how they will comply with the program for covered drugs and sharps. They can do this by either:
1) Becoming an independent “program operator,” or
2) Joining a stewardship organization that serves as a “program operator” on behalf of a collective of covered entities.
In either case, the program operator will design a stewardship program and submit their stewardship plan to state regulators for approval. The stewardship plans must meet all requirements of SB 212, as well as regulatory requirements that have yet to be finalized.
SB212 Regulations will be effective January 1, 2021
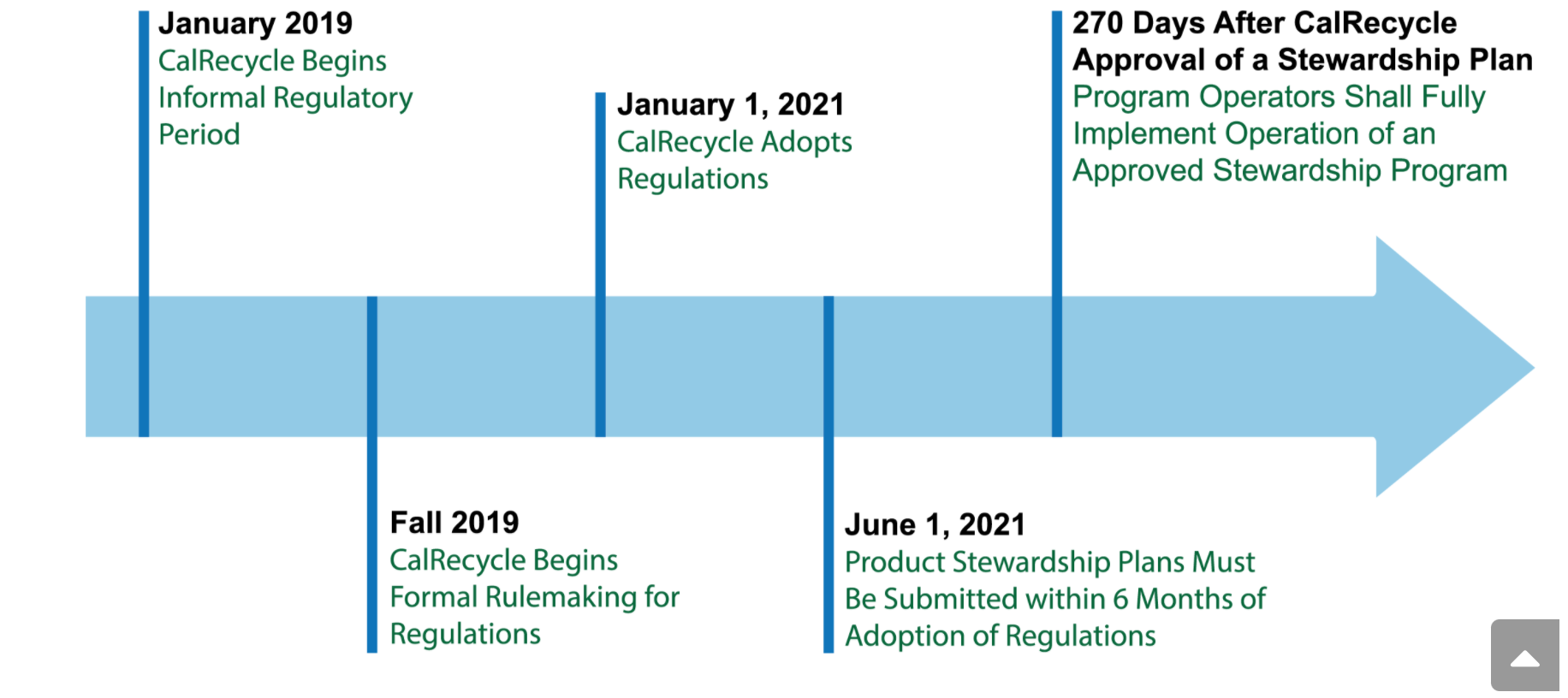
First signed into law in September, 2018, SB 212 directs California’s Department of Resources Recycling and Recovery (CalRecycle) to adopt the regulations effective January 1, 2021. According to its timetable, stewardship plans must be submitted to CalRecycle within six months after the regulations are formally adopted. Program operators must fully implement their approved stewardship programs within 270 days after stewardship plan approval.
CalRecycle provides a great list of resources for more information about SB 212. It also highlights the department’s rule-making efforts. Another great resource is the California’s Department of Health’s list of home-generated sharps and pharmaceutical collection locations.
Why is SB 212 necessary?
According to the CalRecycle, SB 212 and its associated regulations will likely lead to many positive public health and environmental benefits.
Examples of these include a reduction in:
- Needle-stick injuries in waste facilities and among sanitation workers
- Accidental poisonings from unused medications stockpiled in homes
- Prescription drug abuse, and
- Pharmaceutical products ending up in sewers and landfills.
In light of the problems created by pharmaceuticals and sharps waste that either go unused or are improperly disposed, California lawmakers passed SB 212.
This law applies an “Extended Producer Responsibility” approach to safely manage this waste stream. California is the first state to have such a law. It requires state-wide stewardship programs for both pharmaceuticals and home-generated sharps waste.
What is Extended Producer Responsibility (EPR)?
EPR “extends” the responsibility of sharps and drug disposal to the producers of those products. Not to consumers and local governments.
The California Product Stewardship Council, which sponsored SB 212, said that doing so would:
- Put responsibility on the producer, so they can make informed marketing and design decisions
- Integrate the cost of waste treatment and disposal into the total cost of the products
- Help keep environmental impact of products top of mind, for producers, generators AND consumers.
What About Small Business Sharp Generators?
Businesses who produce medical waste such as sharps will continue to abide by California’s Medical Waste Management Act.
They can contract with a medical waste transport and disposal company. Or, they can use a mail-back service, which may be a more economical option for smaller medical waste generators (those with 30 lbs. or less a month).
MERI offers a nationwide medical waste mail-back service. Visit us online or call us at 608-257-7652 to learn more.
###



